nitrogen how many electrons|Chemistry of Nitrogen (Z=7) : iloilo Oxygen makes up 21% of the atmosphere by volume. This is halfway between 17% . 49's Results - UK Last: 20 50 100 Month: January February March April May June July August September October November December Year: 2024 2023 2022 2021 2020 2019 2018 2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2007 2006 2005 2004 2003 2002 2001 2000 1999 1998 1997 1996
PH0 · Protons, Neutrons, Electrons for Nitrogen (N, N3−)
PH1 · Nitrogen Electron Configuration (N) with Orbital Diagram
PH2 · Nitrogen (N)
PH3 · Nitrogen
PH4 · Chemistry of Nitrogen (Z=7)
PH5 · 8.9.2: Chemistry of Nitrogen (Z=7)
There are 37 flights every week to Puerto Princesa. At AirAsia, we want to make flying convenient and affordable for everyone. That's why we offer 108 flights every week to Puerto Princesa so that you can travel on your own terms and explore Puerto Princesa to your heart's content.
nitrogen how many electrons*******Element Nitrogen (N), Group 15, Atomic Number 7, p-block, Mass 14.007. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Members of a group typically have similar properties and electron configurations in their outer shell. .nitrogen how many electrons Chemistry of Nitrogen (Z=7) Oxygen makes up 21% of the atmosphere by volume. This is halfway between 17% .
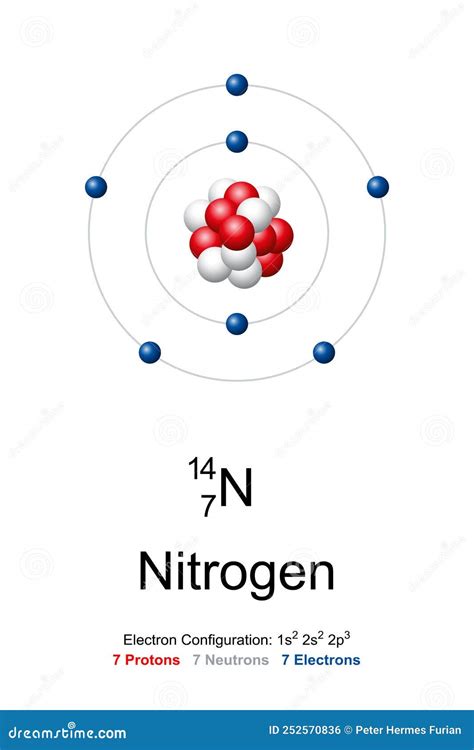
The arrangements of electrons above the last (closed shell) noble gas. Melting . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an atom to . The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of . electron configuration: 1s 2 2s 2 2p 3: History. About four-fifths of Earth’s atmosphere is nitrogen, which was isolated and recognized as a specific substance during early investigations of the air. . Free .z. It, therefore, has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), .nitrogen how many electronsNitrogen has seven protons and seven neutrons in its nucleus, and seven electrons in two shells. It is located in group fifteen, period two and block p of the periodic table. .

The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. Therefore, the number of electrons in neutral atom of Nitrogen is 7. Each electron is influenced by the . Nitrogen has a total of 5 valence electrons, so doubling that, we would have a total of 10 valence electrons with two nitrogen atoms. The octet requires an .
Nitrogen is the seventh element of the periodic table with a total of 7 electrons. When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and .The electron configuration of nitrogen is thus 1s 2 2s 2 2p 3. At oxygen, with Z = 8 and eight electrons, . How many valence electrons are found in the ground state electron configuration for Element 114? Answer: 1s .Atomic number, atomic weight and charge of nitrogen ion. During the formation of a bond, the last shell of nitrogen receives three electrons and turns into a nitride ion (N 3- ). That is, nitrogen is an anion element. N + .
In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Nitrogen is [He] 2s2 2p3. Possible oxidation states are +1,2,3,4,5/-1,2,3. Many industrially important .Chemistry of Nitrogen (Z=7) Nitrogen is found to have either 3 or 5 valence electrons and lies at the top of Group 15 on the periodic table. It can have either 3 or 5 valence electrons because it can bond in the outer 2p and 2s orbitals. Molecular nitrogen (\(N_2\)) is not reactive at standard temperature and pressure and is a colorless and odorless gas.
Valence electrons. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal . Contributions & Attributions. 4.7: Ions - Losing and Gaining Electrons is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. Atom may lose valence electrons to obtain a lower shell that contains an octet. Atoms that lose electrons acquire a positive charge as a result.
When we write the electron configuration of N the first two electrons go in the 1s orbital. As 1s can only hold 2 electrons and the other next two electrons for Nitrogen (N) go in the 2s orbital. The three electrons that are remained will go in the 2p orbital. Therefore the N electron configuration is 1s 2 2s 2 2p 3.Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the . For example, carbon has a valence of 4 and has 4 valence electrons, nitrogen has a valence of 3 and has 5 valence electrons, and oxygen has a valence of 2 and has 6 valence electrons. Hydrogen is an important special case with a single valence electron and a valence of 1. Interestingly, for each of these atoms, the number of bonds .The number of unpaired electrons in the last orbit of an element is the valency of that element. As we know, the correct electron configuration of nitrogen in ground state will be 1s 2 2s 2 2p x1 2p y1 2p z1. Here, the nitrogen atom has three unpaired electrons. Therefore, the valency of nitrogen is 3. 4th shell can hold 32 electrons. Now the atomic number of Nitrogen (N) is 7. Hence nitrogen element has the electrons arrangement 2, 5. This electron arrangement indicates that the outermost orbit of . The valence electrons of nitrogen in its compounds are all sp³ hybridized orbitals. The formal charge on N is usually -1 for an anion, 0 for a neutral compound, and +1 in cations. A nitrogen atom with a formal charge of -3 would correspond to a nitride ion, . Five. The number of valence electrons is the number of electrons in the outer shell, that the atom uses for bonding. Nitrogen has 5 electrons in its n=2 (outer) shell. There is a quick way of identifying the number of valence electrons - it is the same as the Group number (not for d-block elements, though). Nitrogen is in Group 5, so it . electron configuration: 1s 2 2s 2 2p 3: History. About four-fifths of Earth’s atmosphere is nitrogen, which was isolated and recognized as a specific substance during early investigations of the air. . Free nitrogen is found in many meteorites; in gases of volcanoes, mines, and some mineral springs; in the Sun; and in some stars and nebulae
In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Nitrogen. From the Periodic Ta.
The simplest compound of nitrogen is molecular nitrogen, N2 N 2. The two nitrogen atoms are bonded together by a triple bond, consisting of a σ σ and two π π bonds. Molecular nitrogen, N2 N 2 [8] A common nitrogen-containing molecule is ammonia ( NH3 N H 3 ), which is analogous to methane ( CH3 C H 3 ). In ammonia the nitrogen atom is .
Excellent & Newest Movies, TV Shows Watch Online on 123movies. No ADs No Registration Online Streaming Free
nitrogen how many electrons|Chemistry of Nitrogen (Z=7)